A Chinese mpox vaccine with fully independent intellectual property rights was approved for clinical trials on Dec 10.
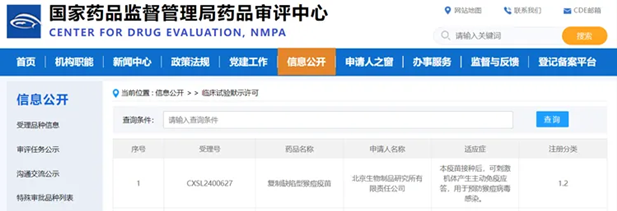
The Center for Drug Evaluation, NMPA, has approved clinical trials of the mpox vaccine co-developed by Beijing Institute of Biological Products Co Ltd under Sinopharm. [Photo/sinopharm.com]
Classified as a category 1.2 innovative vaccine, it is co-developed by Sinopharm’s Beijing Institute of Biological Products Co Ltd and the National Institute for Viral Disease Control and Prevention under the Chinese Center for Disease Control and Prevention.
The breakthrough vaccine is expected to play a critical role in China’s prevention and control of diseases caused by the mpox virus.
The WHO declares mpox a public health emergency of international concern on Aug 14, 2024. [Photo/sinopharm.com]
Since 2022, mpox outbreaks have surged globally. On Aug 14 this year, the World Health Organization (WHO) declared mpox a public health emergency of international concern, its highest-level global alert. This is the second PHEIC determination in two years related to mpox.
Vaccination remains a key measure for preventing the spread of the mpox virus. However, a vaccine has yet to be made available on the Chinese market. Preclinical studies of this newly developed vaccine have demonstrated robust safety and efficacy, providing strong immune protection against mpox in various animal models, including non-human primates.
Looking ahead, Sinopharm and its affiliated enterprises will continue to prioritize major national needs and public health. They aim to accelerate subsequent research and development of the mpox vaccine and contribute to the building of a global community of health for all.
Address: Sinopharm Plaza, No 20 Zhichun Road, Haidian district, Beijing
Postcode: 100191
Tel: 86-10-82287727
Fax: 86-10-62033332
- Sinopharm Group Co Ltd (Sinopharm Holding)
- China National Biotec Group Company Ltd
- China State Institute of Pharmaceutical Industry
- China Sinopharm International Corporation
- Reed Sinopharm Exhibitions Co Ltd
- China Traditional Chinese Medicine Holdings Co Ltd
- Shanghai Shyndec Pharmaceutical Co Ltd
- Sino Pharmengin Corporation
- China National Pharmaceutical Investment Co Ltd
- Sinopharm Group Finance Co Ltd
- China National Medicines Corporation Ltd
- China National Accord Medicines Corporation Ltd
- Beijing Tiantan Biological Products Co Ltd
- Chongqing Taiji Industry (Group) Co Ltd
- China Pharmaceutical Industry Association
- China National Narcotic Drugs Association
- China Association of Pharmaceutical Commerce
- China Pharmaceutical Innovation and Research Development Association
- China Association of Medicine Devices Industry
- China Association of Traditional Chinese Medicine
- China Pharmaceutical Culture Society




